Research
Adult tissue stem cells have a special capacity for self-renewal, differentiation, and lifelong persistence in a variety of tissues. These properties are governed by the chromatin state of the cells in which abrogation of stem cell chromatin homeostasis leads to cellular malfunction and diseases such as cancer. The mammalian intestine is the interface between dietary exposure and nutrient uptake. Intestinal stem cells (ISCs) reside at the base of the crypt and form the apex of cell lineage specification along the single-layered intestinal epithelium. ISCs drive rapid renewal of the epithelium and dynamically remodel tissue composition in response to environmental stimuli. They play a pivotal role in responding to dietary signals and nutrient cues. ISCs have demonstrated the ability to adapt to diverse diets. Our research objective is to molecularly define how stem cells modulate homeostasis along the metabolic-transcriptional axis.

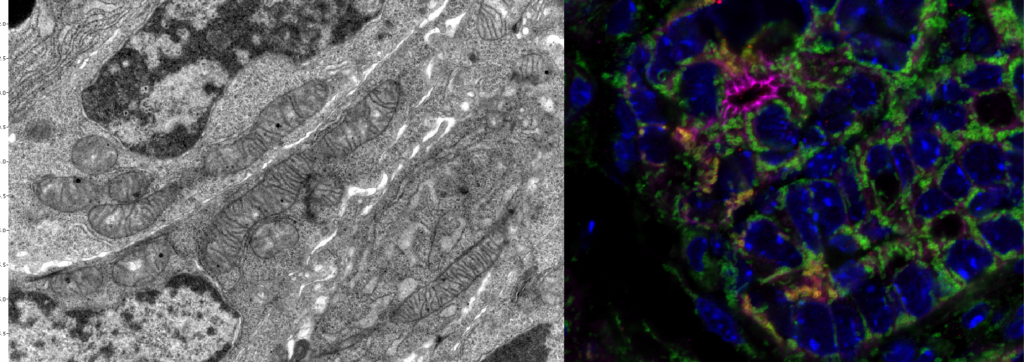
Metabolism underlies all cellular behavior. It must meet energy demands and inform the cell of the environment and nutrient status. Metabolites serve as signaling molecules and substrates to modify histones and DNA for chromatin remodeling, modulating cell function. Mitochondria are the dominant metabolic organelle that sense sugars, lipids, and amino acids, integrating nutritional information and signaling to the rest of the cell. Ongoing projects examine the mitochondrial crosstalk with the nuclear components.
Chromatin remodeling enables adaptation to different environmental conditions. It relies on metabolic products to modify histones and activate epigenetic regulators for transcription factor access to DNA. Utilizing the self-renewal and differentiation properties of ISCs, we leverage dietary interventions to understand how stem cells adapt intrinsic metabolic functions from external nutrient signals to affect epigenetic change. We test how metabolic states remodel chromatin and alter stem cell function towards the pathogenesis of cancer.
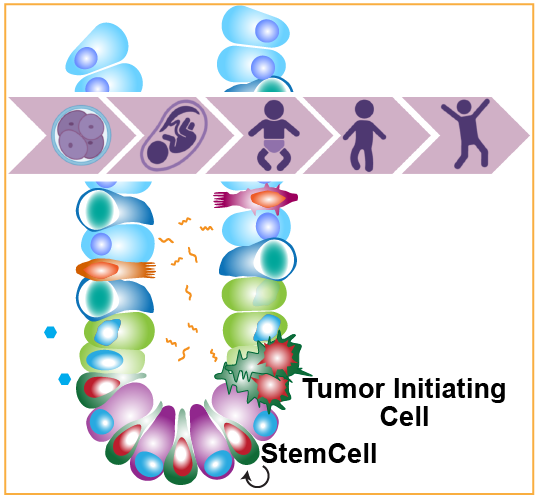
Early life exposures play a significant role in shaping health and disease susceptibility, leading to increased risk of metabolic, inflammatory, and degenerative pathologies. Mature adult ISCs sustain continuous life-long adaptations to diet and the intestinal microbial milieu to preserve tissue stability and prevent sequelae leading to diseases such as inflammatory bowel disease (IBD) or sporadic cancer. ISC’s initial specification in the embryo and trajectory of development is influenced early in life by a maternal environment that provides extrinsic patterning cues and nutrients to influence cell, tissue, and system maturation. Despite how fundamental these phases of development are, little is known about how durable programmable molecular mechanisms are established or how adverse maternal environmental exposure is maintained in a stable and homeostatic manner within ISCs. We are examining the developmental programming of adult ISCs to understand how early patterning affects the later risk of disease.